
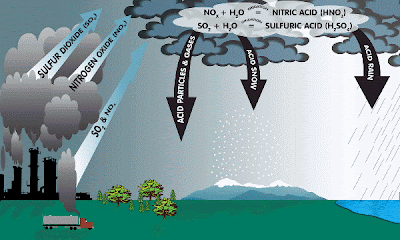
Acid rain is a human-related phenomena. Since our industries are so fond of burning fossil fuels (coal and oil) they tend to release a lot of sulfur into the air. This sulfur combines with the oxygen already present in the air to form sulfur dioxide (SO2).
Also, since we like to drive big fancy cars rather than ride bikes or walk, we cause the formation of nitrogen oxides (NO or NO2 or NO 3, etc) in air from burning gasoline.
CHEMICAL REACTIONS:
Formation of SO2 and nitrogen oxides (NO & NOx): ![]()
S(in fuel) + O2 -------> SO2
N2 + O2 -------> 2NO
NO + .5O2 -----> NO2
Formation of hydrogen peroxide
N2 + O2 -------> 2NO
NO + .5O2 -----> NO2
Formation of hydrogen peroxide
VOC + sunlight + HO2 (in air) -------> H2O2
***VOC = Volatile Organic Compound ***
Formation of acids :
***VOC = Volatile Organic Compound ***
Formation of acids :
SO2 + H2O2 and O3 (in clouds) -------> H2SO4 SO2+ OH + O2 (in air) --------------> H2SO4
SO2 + Oxidants (from wet surfaces) --> H2SO4
NOx + sunlight + OH (air) ------------> HNO3
SO2 + Oxidants (from wet surfaces) --> H2SO4
NOx + sunlight + OH (air) ------------> HNO3
EFFECTS OF ACID RAIN
Also, the leaves of many plants and trees can be severely damaged by acidic precipitation. It is believed that acid rain leaches calcium and magnesium from the soil. This causes a decrease in the ratio of calcium to aluminum in the soil, which stimulates the uptake of aluminum by roots. The uptake of aluminum by trees and plants can be destructive. Finally, in cites and towns all over the world, stone structures, such as buildings, ancient ruins, etc are being deteriorated by the corrosive effects of acidic rainfall.







![Equations to find [OH] and [H3O} using Kw](http://www.chem.purdue.edu/gchelp/howtosolveit/Equilibrium/EquilibriumArt/Kwequations.gif)
